Is Nitrogen Monoxide an Element Compound or Mixture
Hence vinegar is a mixture not a compound. Therefore it is a mixture.

Is It A Compound Element Or Mixture Flashcards Quizlet
If it is a substance with a chemical formula it is a compound.
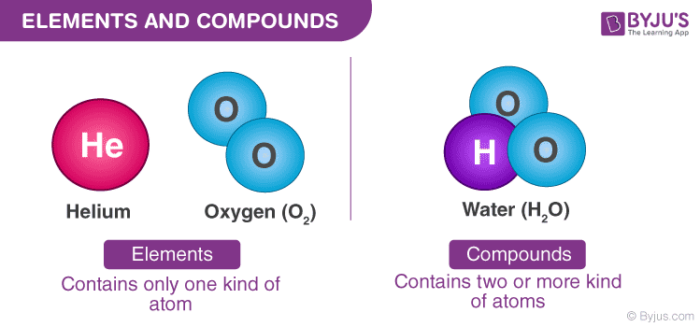
. If it is neither it is a mixture. A pure substance is a matter that consists of 1 type of element or 1 type of substance. It has a boiling point bp of 1518C at 1 atm and molecular weight of 300 g mol 1Because it has an odd number of electrons NO is a very reactive molecule a so-called radical Greenwood and.
2 or more atoms mixed together. Write the formula for each compound carbon monoxide. Nitrogen oxide is a compound because it is an atom of Nitrogen and an atom of Oxygen bonded together.
Element is a matter that is purely consist of one type of atom. Terms in this set 35 iron. Is it a compound element or mixture.
The chemical name for nitrogen monoxide. All alloys are mixtures. It is a colourless odourless tasteless toxic gas.
Terms in this set 40 iron. It is a diatomic element because it is composed of two nitrogen atoms chemically bonded. It is an inert gas under standard temperature and pressure.
Compound Fe₂O₃ table sugar. A compound containing one atom of zinc and two atoms of chlorine. There is no such sodium chloride element in the periodic table.
Why is carbon considered an element and not a compound. Carbon monoxide is a one-carbon compound in which the carbon is joined only to a single oxygen. 2 elements joined together.
There is no chemical reaction between the components and there are no chemical links between them. Its a compound that consists only of carbon dioxide molecules and it has consistent composition throughout the sampling area. In chemistry mixture is a matter that consists of 2 or more types of atoms that is able to be broken down PHYSICALLY.
Is iron an element compound or mixture. Is it a compound element or mixture. Nitrogen is a colorless odorless and tasteless gas.
Pure nitrogen is not a mixture therefore is neither homogeneous nor heterogeneous. Carbon is the only element that can form so many different compounds because each carbon atom can form four chemical bonds to other atoms and because the carbon atom. Mixtures can be homogeneous or heterogeneous.
Element Compound Homogeneous mixture Heterogeneous mixture an alloy of gold and tin titanium metal a substance containing only titanium atoms carbon monoxide a substance containing carbon and oxygen atoms in a fixed 11 ratio phosphorus trichloride substance containing phosphorus and chlorine atom in a fixed ratio Earths atmosphere which contains. Air Is A Mixture For The Following Reasons. What element forms an ion with an electronic configuration of 1s 2s 2p 3s 3p 3d1045-4p or Kr and a -2 charge.
Enter the full electron configuration for P. The concentration of acetic acid present in vinegar can be 4 5 6 or even higher. Compound NO oxygen.
There are only 118 elements on earth. Air consists of various elements and compounds including nitrogen oxygen argon carbon dioxide hydrogen water vapor and many other. If it is a substance with a chemical formula it is a compound.
If you take a sample of a pure substance it should have constant composition no matter. This makes air eligible to be considered a mixture. A compound is composed of two or more elements chemically bonded.
A mixture is a combination of two or more substances that have not undergone a chemical reaction. Salt is made of two different atoms so its not a pure element. Chemistry questions and answers.
If it is one item found on the periodic table it is an element. No salt is not an element. Nitric oxide or nitrogen monoxide NO is a colorless gas.
A vinegar contains water and acetic acid. Yes carbon dioxide is a pure substance. Compound C₁₂H₂₂O₁₁ nitrogen monoxide.
2 or more atoms same or different joined together. Yes compound also contains two or more elements but compound always has a definite ratio of composition. Nitrogen is an element not a compound.
Air is primarily composed of nitrogen 70 and oxygen 20 with tiny amounts of carbon dioxide carbon monoxide helium and traces of many other gases. 2 atoms joined together. If it is neither it is a mixture.
Nitrogen N2 CID 947 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazards. Is carbon monoxide an element a compound a heterogeneous mixture or a homogeneous mixture. Nitrogen is ranked as the seventh-most-abundant element.
Pure iron is an element with an atomic number of 26. This means that they have uniform appearance. All alloys are mixtures.
Dont forget any solution is a homogenous mixture. With heterogeneous mixtures you can see different substances mixed. However the iron that we see in everyday life is usually an iron alloy which is a mixture of the iron element with other metallic elements.
Is NO a mixture compound or element. All alloys are mixtures. It is found in nature as nitrogen gas also called dinitrogen with the chemical formula N_2 and makes up 78 of the atmosphere.
Some examples of elements are Oxygen O2 Hydrogen H2 or Nitrogen N2. If it is a substance with a chemical formula it is a compound. Give the symbol for the element.
If it is neither it is a mixture. It means that there is no definite ratio of composition in vinegar. Bange in Nitrogen in the Marine Environment Second Edition 2008 21 Climatic and biogeochemical relevance.

Calculate Enthalpy Example Problems Solutions 4 Chemical Equation Problem And Solution Chemistry

Yttrium Barium Copper Oxide Yba2cu3o7 Powder Funcmater In 2021 Liquid Nitrogen Inorganic Compound Copper

1 5 Classification Of Matter Chemistry Libretexts

Nitrogen Monoxide An Overview Sciencedirect Topics

Classify The Following Into Elements Compounds And Mixtures A Sodium B Soil C Sugar Sol Youtube

Element Compound And Mixture Chemsitry Youtube
Difference Between Compound And Mixture Definition Characteristics Types Of Bonding

Ks4 Chemistry Ionic Bonding Ionic Bonding Electron Configuration Covalent Bonding

Pure Substances And Mixtures Elements Compounds Classification Of Matter Chemistry Examples Youtube

Elements Compounds And Mixtures

Selina Concise Chemistry Class 7 Icse Solutions Chapter 3 Elements Compounds And Mixtures Learn Cram Https Chemistry Class Chemistry Compounds And Mixtures

Four Covalent Bonds Carbon Has Four Valence Electrons And Here A Valence Of Four Each Hydrogen Atom Has One Vale Covalent Bonding Chemical Bond Ionic Bonding

Position Carbon Series Reactivity Metals 2 Oxygen Metal Carbon Dioxide

Four Covalent Bonds Carbon Has Four Valence Electrons And Here A Valence Of Four Each Hydrogen Atom Has One Vale Covalent Bonding Chemical Bond Ionic Bonding

Pure Substances And Mixtures Elements Compounds Classification Of Matter Chemistry Examples Youtube

Unsur Senyawa Pengertian Perbedaan Contoh Beserta Gambarnya Chemistry Classroom Chemistry Teaching Chemistry

Comments
Post a Comment